
CAC2 Member Blog–Joining Forces to Bring New Therapies to Children with Cancer
By CAC2 Member Cesare Spadoni (aPODD). “Pharma and biotech companies do not develop drugs for kids with cancer!” How many times have we heard these

By CAC2 Member Cesare Spadoni (aPODD). “Pharma and biotech companies do not develop drugs for kids with cancer!” How many times have we heard these
Over 100 CAC2 members and guests gathered for a special online event on Friday, July 17, 2020 to attend our 8th Annual Summit Speaker Program

Update from our colleagues at the Alliance for Childhood Cancer (July 13, 2020): The House Appropriations Committee agreed to fully fund the Childhood Cancer STAR
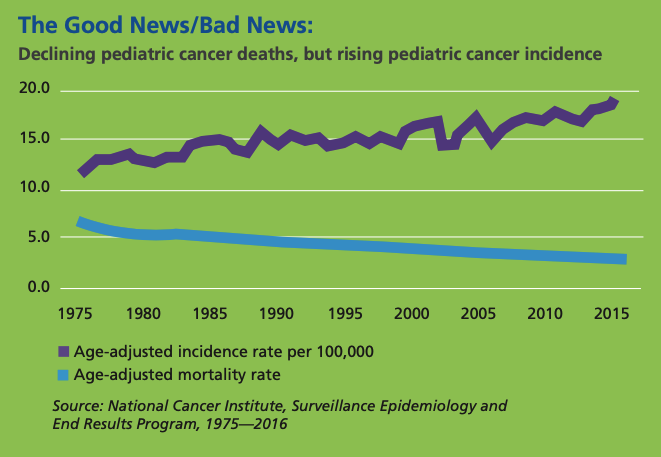
I frequently speak of viewing childhood cancer like a bicycle wheel with multiple spokes emanating from the hub in the center. Each spoke represents a

The CAC2 Survivorship Working Group assembled this document to help categorize survivorship issues for the community. It is by no means an exhaustive list, but it

Our June CAC2 All-Member webinar explored the world of clinical trials and helped us understand the important role of clinical trial navigation, a topic that is
On June 16, 2020, the Food and Drug Administration extended the indication of gemtuzumab ozogamicin (MYLOTARGTM, Wyeth Pharmaceuticals LLC) for newly-diagnosed CD33-positive acute myeloid leukemia
The American Association for Cancer Research (AACR) and the U.S. Food and Drug Administration (FDA) recorded a listening session on May 27, 2020 for patient

Our May CAC2 All-Member webinar focused on how pediatric cancer study plans are developed. CAC2 Member Kelli Wright (CureSearch) introduced Dr. Brenda Weigel from University
Day One Biopharmaceutical, a start-up biotech company founded with a focus on developing therapies for children with cancer through drug repurposing announced its debut in
