
Guest Blog–The Fit for Filing Group at ACCELERATE
Blogged with permission and thanks by the ACCELERATE Fit for Filing Working Group The ACCELERATE PLATFORM’S Fit for Filing (FFF) group was formed in 2019 to explore

Blogged with permission and thanks by the ACCELERATE Fit for Filing Working Group The ACCELERATE PLATFORM’S Fit for Filing (FFF) group was formed in 2019 to explore

The U.S. Food and Drug Administration issued a draft guidance that, when finalized, will provide the agency’s perspective on the ethical considerations for including and

Children, adolescents, and young adults (AYAs) with newly diagnosed rare tumors are now eligible to enroll in the CCDI Molecular Characterization Initiative. Rare tumors are
The Molecular Targets Platform is an NCI-supported instance of the Open Targets Platform with a focus on preclinical pediatric oncology data. It is a tool

Update from our colleagues at the Alliance for Childhood Cancer (July 28, 2022): The Senate Appropriations Committee included language to fund the Childhood Cancer STAR

The National Cancer Institute’s new Molecular Characterization Initiative (MCI) fosters data sharing in childhood cancer research. The program is expanding comprehensive molecular characterization of tumors

The Childhood Cancer Data Catalog is a searchable database of National Cancer Institute and other pediatric cancer resources and is part of NCI’s Childhood Cancer Data

During last week’s Childhood Cancer Action Day, legislation to reauthorize the Childhood Cancer STAR Act was introduced in both chambers of Congress! The original STAR Act

The Alliance for Childhood Cancer sends its thanks for a fantastic Action Day on April 28! Nearly 300 advocates from 39 states and Washington, DC
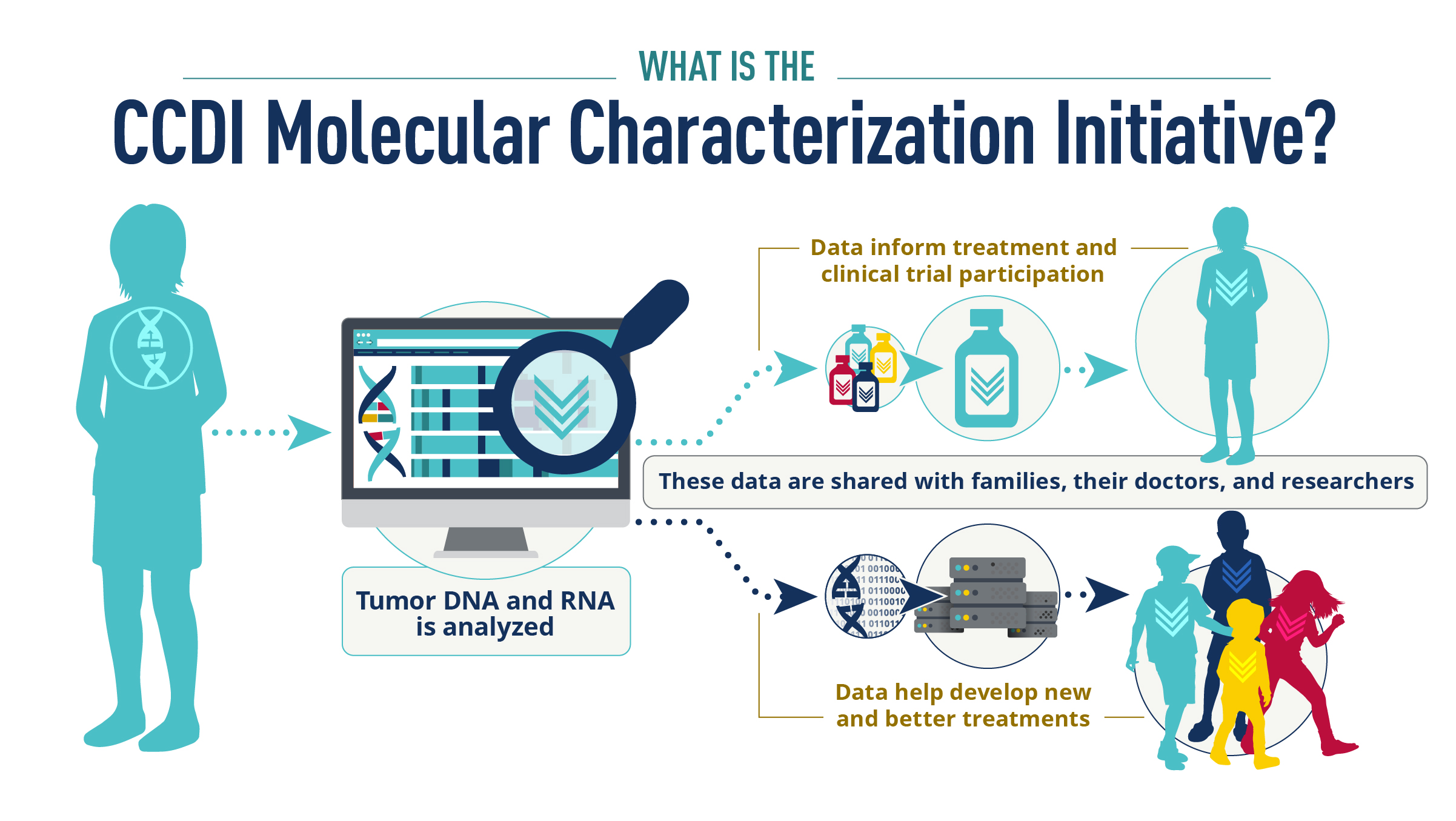
The National Cancer Institute’s new Molecular Characterization Initiative (MCI) fosters data sharing in childhood cancer research. The program currently offers comprehensive molecular characterization of tumors
